CONTENIDOS DE LA ASIGNATURA
BLOQUE 1: EL TRABAJO CIENTÍFICO
BLOQUE 2: ESTUDIO DEL ESTADO GASEOSO
BLOQUE 3: EL ÁTOMO
BLOQUE 4: ELEMENTOS Y COMPUESTOS
BLOQUE 5: REACCIONES QUÍMICAS
BLOQUE 6: LAS FUERZAS Y EL MOVIMIENTO
Material two
*SECOND: CHARLES´S LAW
*THIRD: GAY-LUSSAC´S LAWPART 2: STUDY OF THE GAS STATE
*GAS LAWS VIDEOS
*FIRST: BOYLE´S LAW*SECOND: CHARLES´S LAW
*SUMMARY OF GASEOUS STATE
*MATERIAL TO STUDY AT HOME*
*BOYLE´S LAW PROBLEMS *CHARLES´S LAW PROBLEMS
*GAY-LUSSAC´S LAW PROBLEMS
*COMBINED GAS LAW PROBLEMS (WITH THE SOLUTIONS)
Boyle's Law Concept Questions
Gas Laws Review Quiz
MÁS EN ESPAÑOL
* HOMOGENEUS MIXTURES OR SOLUTIONS
* TYPES OF SOLUTIONS.SOLUBILITY
* MORE CONCENTRATION EXERCISES WITH SOLUTION
* MIXTURES AND SOLUTIONS VS CHEMICAL REACTIONS
* TYPES OF SOLUTIONS.SOLUBILITY
* MORE CONCENTRATION EXERCISES WITH SOLUTION
* MIXTURES AND SOLUTIONS VS CHEMICAL REACTIONS
PART 3: ATOMS
Taken from this LINK
THE STANDAR MODELOF PARTICLE PHYSICS
2.- ORIGINS OF ELEMENTAL NAMES
3.- THE PERIODIC TABLE SONG
*READING COMPREHENSION: ATOMIC HISTORY
* BUILD AN ATOM
*CHEMICAL BOND1
*CHEMICAL BOND2
*DIFFERENT MATERIALS TO STUDY CHEMICAL BONDS
2.- ORIGINS OF ELEMENTAL NAMES
3.- THE PERIODIC TABLE SONG
*READING COMPREHENSION: ATOMIC HISTORY
* BUILD AN ATOM
*CHEMICAL BOND1
*CHEMICAL BOND2
*DIFFERENT MATERIALS TO STUDY CHEMICAL BONDS
FORMULA WRITING AND NOMENCLATURE
OF INORGANIC COMPOUNDS
* Naming Inorganic CompoundsPART 5: CHEMICAL REACTIONS.
THE MOLE
* STOICHOMETRY PRACTICE PROBLEMS
* CHEMICAL REACTIONS AND EXERCISES
* CHEMICAL REACTIONS EXERCISES
* CONSERVATION OF MASS WORKSHEET
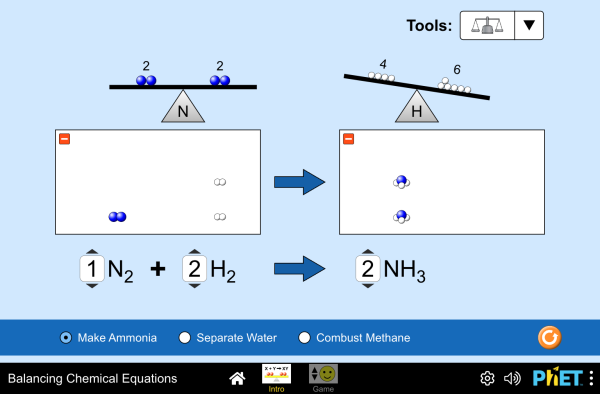
AJUSTE DE REACCIONES QUÍMICAS
EXERCISES
1.- In the following chemical reaction: H2SO4 + K → K2SO4 + H2
Calculate the amount of K2SO4 that can be obtained from 150 g of potassium.
2.- Calculate how many grams of aluminium fluoride are obtained when 150 g of aluminium reacts with a defined quantity of fluorine.
3.- With the following chemical reaction:
C5H11OH + O2 → CO2 + H2O
answer the following questions:
a) Write down the balanced chemical equation.
b) How many moles of water are formed from each mole of oxygen used?
c) How many moles of oxygen are needed to burn one mole of alcohol?
d) How many grams of carbon dioxide are produced from each mole of alcohol burned?
e) How many grams of carbon dioxide are produced from 2 g of alcohol burned?
4.- Indica si son verdaderas o falsas las siguientes afirmaciones. En las que sean falsas pon un ejemplo para demostrarlo:
a) En todas las reacciones químicas se conserva el número de átomos.
b) En todas las reacciones químicas se conserva el número de moléculas.
c) En todas las reacciones químicas se conserva la masa.
d) En todas las reacciones químicas hay el mismo número de sustancias en los reactivos y en los productos.
* CHEMICAL REACTIONS AND EXERCISES
* CHEMICAL REACTIONS EXERCISES
* CONSERVATION OF MASS WORKSHEET
AJUSTE DE REACCIONES QUÍMICAS
EXERCISES
1.- In the following chemical reaction: H2SO4 + K → K2SO4 + H2
Calculate the amount of K2SO4 that can be obtained from 150 g of potassium.
2.- Calculate how many grams of aluminium fluoride are obtained when 150 g of aluminium reacts with a defined quantity of fluorine.
3.- With the following chemical reaction:
C5H11OH + O2 → CO2 + H2O
answer the following questions:
a) Write down the balanced chemical equation.
b) How many moles of water are formed from each mole of oxygen used?
c) How many moles of oxygen are needed to burn one mole of alcohol?
d) How many grams of carbon dioxide are produced from each mole of alcohol burned?
e) How many grams of carbon dioxide are produced from 2 g of alcohol burned?
4.- Indica si son verdaderas o falsas las siguientes afirmaciones. En las que sean falsas pon un ejemplo para demostrarlo:
a) En todas las reacciones químicas se conserva el número de átomos.
b) En todas las reacciones químicas se conserva el número de moléculas.
c) En todas las reacciones químicas se conserva la masa.
d) En todas las reacciones químicas hay el mismo número de sustancias en los reactivos y en los productos.
THE MOLE
To start with, you must be very clear about what exactly does a mole represent. The mole is the unit of measurement in the International System of Units (SI) for amount of substance. 1 mole in quantity, that implies that the matter under consideration contains exactly 6.022 * 1023 number of particles (atoms, molecules, ions, electrons or any other elementary entities).
Though mole is defined as a number but it’s not limited to that in calculations. It has various other equivalent definitions with the only difference from each other being that they define mole for different states of matter and at different conditions.
Though mole is defined as a number but it’s not limited to that in calculations. It has various other equivalent definitions with the only difference from each other being that they define mole for different states of matter and at different conditions.